Використання методу k-найближчих сусідів для класифікації концентрації препаратів та ризику кардіотоксичності з використанням потенціалів позаклітинних полів та реконструйованих потенціалів дії серцевих клітин
Основний зміст сторінки статті
Анотація
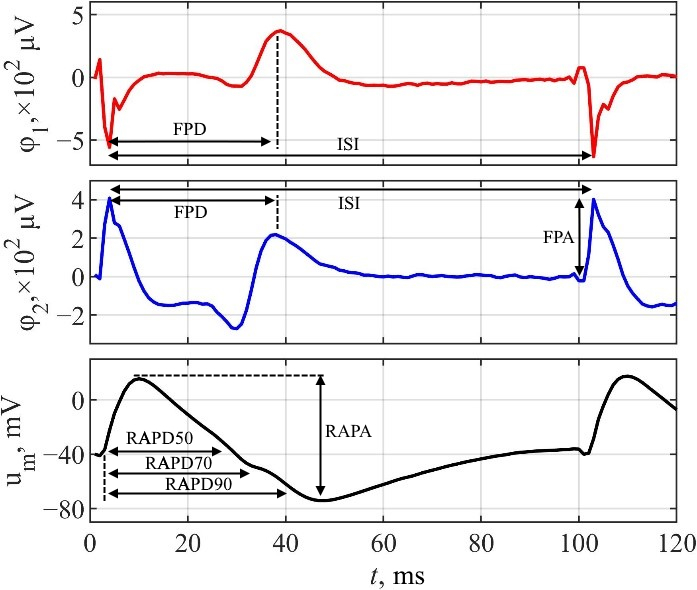
Системи з мікроелектродними решітками (МЕР) важливі для вимірювання позаклітинних потенціалів поля (ПП) клітин серця, що є важливим кроком в оцінці кардіотоксичності. Однак, без модифікації БЕР система здатна реєструвати лише потенціали поля. Це обмежує кількість параметрів для оцінки кардіотоксичності лише параметрами ПП, в той час як параметри потенціалу дії (ПД) залишаються невикористаними. Для вирішення цієї проблеми БЕР системи модифікують, щоб використовувати електро- або оптопорацію для реєстрації локальних позаклітинних потенціалів дії (ЛППД), що дозволяє отримувати сигнали з достовірною морфологію ПД. З іншого боку, існує альтернатива модифікації МЕР систем, що дозволяє уникнути стимуляції клітинної мембрани ⸺ математична реконструкція ПД.
У цьому дослідженні вивчається, як використання додаткових параметрів реконструйованих потенціалів дії (РПД), отриманих з ПП, може підвищити точність таких моделей машинного навчання як k-найближчих сусідів (k-NN) для класифікації концентрацій лікарських препаратів та ризику їхньої кардіотоксичності.
Класифікатор k-NN було натреновано на комбінаціях параметрів ПП та РПД. Перевірка моделей була проведена за допомогою п'ятикратної перехресної валідації та міжканальної валідації. Якість k-NN моделей була оцінена за допомогою таких метрик точності як частота помилок, макро влучність, макро повнота та макро F1-міра.
Результати показали, що включення РПД параметрів до набору ознак підвищило F1-міру моделі k-NN для класифікації концентрації Dymethylsulfoxide (DMSO) до 10.78% порівняно з моделями, які були натреновані виключно на ознаках з ПП.
Блок інформації про статтю

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Автори, які публікуються у цьому журналі, погоджуються з наступними умовами:- Автори залишають за собою право на авторство своєї роботи та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons Attribution License, котра дозволяє іншим особам вільно розповсюджувати опубліковану роботу з обов'язковим посиланням на авторів оригінальної роботи та першу публікацію роботи у цьому журналі.
- Автори мають право укладати самостійні додаткові угоди щодо неексклюзивного розповсюдження роботи у тому вигляді, в якому вона була опублікована цим журналом (наприклад, розміщувати роботу в електронному сховищі установи або публікувати у складі монографії), за умови збереження посилання на першу публікацію роботи у цьому журналі.
- Політика журналу дозволяє і заохочує розміщення авторами в мережі Інтернет (наприклад, у сховищах установ або на особистих веб-сайтах) рукопису роботи, як до подання цього рукопису до редакції, так і під час його редакційного опрацювання, оскільки це сприяє виникненню продуктивної наукової дискусії та позитивно позначається на оперативності та динаміці цитування опублікованої роботи (див. The Effect of Open Access).
Посилання
K. W. Johnson et al., “Artificial Intelligence in Cardiology”, J. Amer. College Cardiol., vol. 71, no. 23, pp. 2668–2679, Jun. 2018. DOI: https://doi.org/10.1016/j.jacc.2018.03.521
P. P. Kanade et al., “MEA-integrated cantilever platform for comparison of real-time change in electrophysiology and contractility of cardiomyocytes to drugs”, Biosens. Bioelectron., p. 114675, Sep. 2022. DOI: https://doi.org/10.1016/j.bios.2022.114675
H. Chen, O. Engkvist, Y. Wang, M. Olivecrona, and T. Blaschke, “The rise of deep learning in drug discovery”, Drug Discov. Today, vol. 23, no. 6, pp. 1241–1250, Jun. 2018. DOI: https://doi.org/10.1016/j.drudis.2018.01.039
J. M. Rivera‐Arbeláez et al., “Automated assessment of human engineered heart tissues using deep learning and template matching for segmentation and tracking”, Bioeng. & Translational Medicine, Apr. 2023. DOI: https://doi.org/10.1002/btm2.10513
W. Guo et al., “Review of machine learning and deep learning models for toxicity prediction”, Exp. Biol. Medicine, Dec. 2023. DOI: https://doi.org/10.1177/15353702231209421
L. Pu, M. Naderi, T. Liu, H.-C. Wu, S. Mukhopadhyay, and M. Brylinski, “eToxPred: a machine learning-based approach to estimate the toxicity of drug candidates”, BMC Pharmacol. Toxicol., vol. 20, no. 1, Jan. 2019. DOI: https://doi.org/10.1186/s40360-018-0282-6
D. Pan, B. Li, and S. Wang, “Establishment and validation of a torsade de pointes prediction model based on human iPSC‑derived cardiomyocytes”, Exp. Therapeutic Medicine, vol. 25, no. 1, Dec. 2022. DOI: https://doi.org/10.3892/etm.2022.11760
H. B. Hayes et al., “Novel method for action potential measurements from intact cardiac monolayers with multiwell microelectrode array technology”, Scientific Rep., vol. 9, no. 1, Aug. 2019. DOI: https://doi.org/10.1038/s41598-019-48174-5
B. Duckert, M. Fauvart, P. Goos, T. Stakenborg, L. Lagae, and D. Braeken, “High-definition electroporation: Precise and efficient transfection on a microelectrode array”, J. Controlled Release, vol. 352, pp. 61–73, Dec. 2022. DOI: https://doi.org/10.1016/j.jconrel.2022.10.001
M. Dipalo et al., “Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy graphene microelectrodes”, Sci. Advances, vol. 7, no. 15, Apr. 2021, Art. no. eabd5175. DOI: https://doi.org/10.1126/sciadv.abd5175
N. G. Ivanushkina, K. O. Ivanko, M. O. Shpotak, and Y. V. Prokopenko, “Reconstruction of action potentials of cardiac cells from extracellular field potentials”, Radioelectronics Commun. Syst., vol. 65, no. 7, pp. 354–364, Jul. 2022. DOI: https://doi.org/10.3103/s0735272722090047
R. Visone et al., “Micro-electrode channel guide (µECG) technology: An online method for continuous electrical recording in a human beating heart-on-chip”, Biofabrication, vol. 13, no. 3, p. 035026, Apr. 2021. DOI: https://doi.org/10.1088/1758-5090/abe4c4
M. Malik, “Drug-Induced qt/qtc interval shortening: Lessons from drug-induced qt/qtc prolongation”, Drug Saf., vol. 39, no. 7, pp. 647–659, Mar. 2016. DOI: https://doi.org/10.1007/s40264-016-0411-3
L. G. J. Tertoolen, S. R. Braam, B. J. van Meer, R. Passier, and C. L. Mummery, “Interpretation of field potentials measured on a multi electrode array in pharmacological toxicity screening on primary and human pluripotent stem cell-derived cardiomyocytes”, Biochem. Biophysical Res. Commun., vol. 497, no. 4, pp. 1135–1141, Mar. 2018. DOI: https://doi.org/10.1016/j.bbrc.2017.01.151
A. A. Kondratyev, J. G. C. Ponard, A. Munteanu, S. Rohr, and J. P. Kucera, “Dynamic changes of cardiac conduction during rapid pacing”, Amer. J. Physiol.-Heart Circulatory Physiology, vol. 292, no. 4, pp. H1796—H1811, Apr. 2007. DOI: https://doi.org/10.1152/ajpheart.00784.2006
S.-W. Hyun, B.-R. Kim, S.-A. Hyun, and J.-W. Seo, “The assessment of electrophysiological activity in human-induced pluripotent stem cell-derived cardiomyocytes exposed to dimethyl sulfoxide and ethanol by manual patch clamp and multi-electrode array system”, J. Pharmacolog. Toxicolog. Methods, vol. 87, pp. 93–98, Sep. 2017. DOI: https://doi.org/10.1016/j.vascn.2017.03.003
P. Pradhapan, J. Kuusela, J. Viik, K. Aalto-Setälä, and J. Hyttinen, “Cardiomyocyte MEA Data Analysis (CardioMDA) – A Novel Field Potential Data Analysis Software for Pluripotent Stem Cell Derived Cardiomyocytes”, PLoS ONE, vol. 8, no. 9, Sep. 2013, Art. no. e73637. DOI: https://doi.org/10.1371/journal.pone.0073637
T. Kaneko et al., “On-chip in vitro cell-network pre-clinical cardiac toxicity using spatiotemporal human cardiomyocyte measurement on a chip”, Scientific Rep., vol. 4, no. 1, Apr. 2014. DOI: https://doi.org/10.1038/srep04670
I. Saini, D. Singh, and A. Khosla, “QRS detection using K-Nearest Neighbor algorithm (KNN) and evaluation on standard ECG databases”, J. Adv. Res., vol. 4, no. 4, pp. 331–344, Jul. 2013. DOI: https://doi.org/10.1016/j.jare.2012.05.007
J. Galvao, B. Davis, M. Tilley, E. Normando, M. R. Duchen, and M. F. Cordeiro, “Unexpected low‐dose toxicity of the universal solvent DMSO”, FASEB J., vol. 28, no. 3, pp. 1317–1330, Dec. 2013. DOI: https://doi.org/10.1096/fj.13-235440
N. Augustin, C. Alvarez, and J. Kluger, “The Arrhythmogenicity of Sotalol and its Role in Heart Failure: A Literature Review”, J. Cardiovascular Pharmacol., Publish Ahead of Print, May 2023. DOI: https://doi.org/10.1097/fjc.0000000000001439
H. Lenhoff, H. Jarnbert-Petersson, B. Darpo, P. Tornvall, and M. Frick, “Mortality and ventricular arrhythmias in patients on d,l-sotalol for rhythm control of atrial fibrillation - A nationwide cohort study”, Heart Rhythm, Aug. 2023. DOI: https://doi.org/10.1016/j.hrthm.2023.08.019
J. Larson, L. Rich, A. Deshmukh, E. C. Judge, and J. J. Liang, “Pharmacologic Management for Ventricular Arrhythmias: Overview of Anti-Arrhythmic Drugs”, J. Clin. Medicine, vol. 11, no. 11, p. 3233, Jun. 2022. DOI: https://doi.org/10.3390/jcm11113233